CRISPR is blurring the line between science fiction and reality
A New World
In 1993, audiences were transported to a universe where dinosaurs roamed the earth once again, thanks to the power of genetic engineering. Jurassic Park captivated imaginations with its thrilling depiction of science's ability to resurrect the past, while also serving as a cautionary tale of its potential dangers. 30 years later, the line between science fiction and reality is beginning to blur.
Colossal Biosciences is working to de-extinct ancient animals - a development they claim will help combat climate change. However, Colossal is just one of many companies powered by CRISPR technology, the most significant advancement in genetic engineering to date. First reported by Jennifer Doudna and Dr. Emmanuelle Charpentier in 2012, CRISPR allows scientists to precisely edit an organism's genome. Their groundbreaking work earned them the Nobel Prize.
CRISPR has been likened to the "hand of God" and even compared to the power of a nuclear bomb. But, in order to fully grasp the impact of CRISPR, it is necessary to explore the inner workings of this gene-editing tool and the multi-billion dollar industry it has created. Together, these insights reveal CRISPR has already begun to change life as we know it.
How CRISPR Works
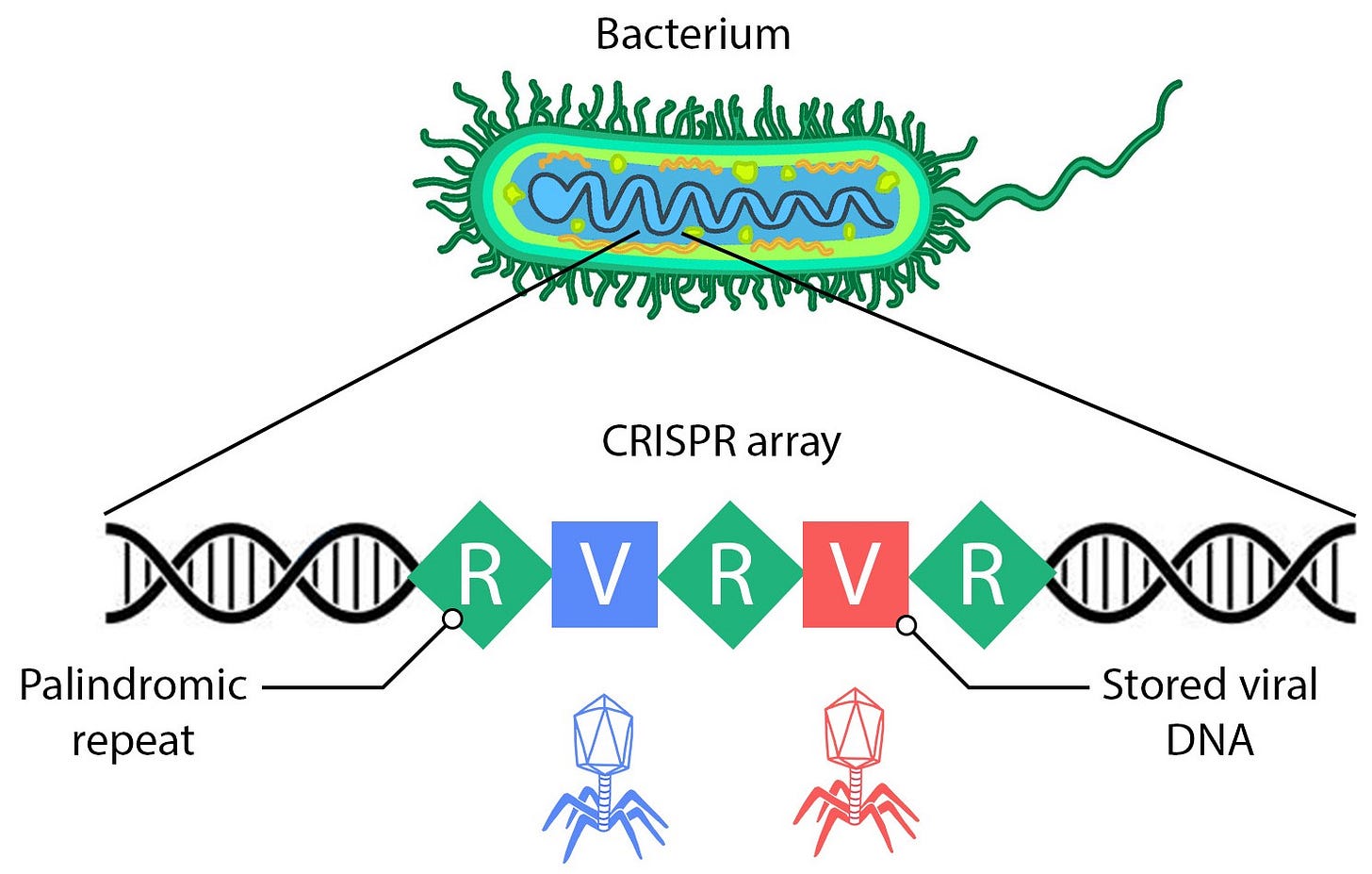
CRISPR-Cas9 was first observed as an immune defense system within bacteria. When a virus attacks, it injects its viral DNA into the bacterium’s cytoplasm. The bacterium then captures fragments of this viral DNA and embeds them into a specific section of its own DNA called a CRISPR array. CRISPR stands for Clustered Regularly Interspaced Palindromic Repeats because this section of bacterial DNA features repeated sequences between the viral information.
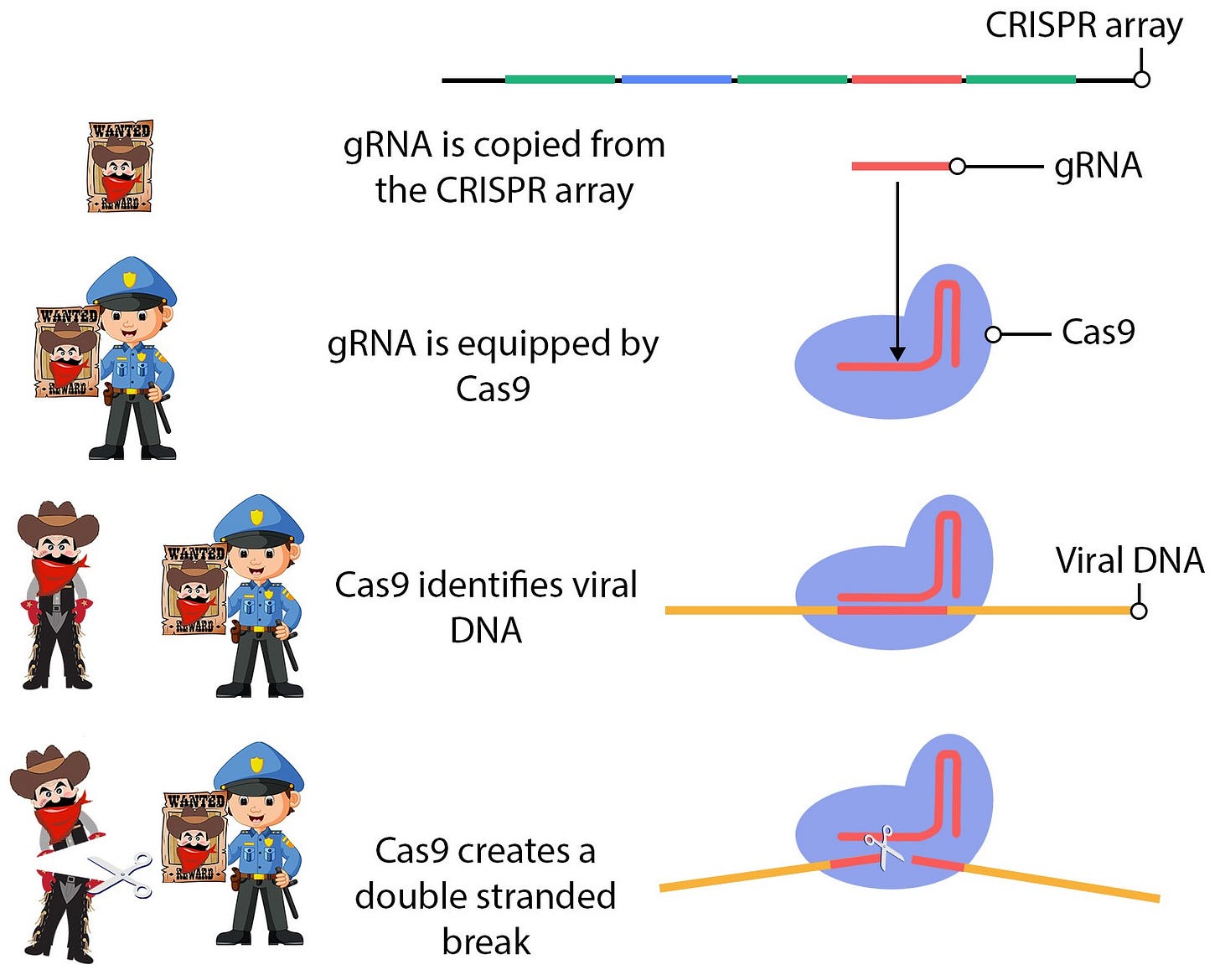
CRISPR arrays function as the bacterium's immune memory, storing information about previously encountered viruses. Guide RNA (gRNA) is copied from each section of the stored DNA and used by a molecular tool called Cas9 to identify and destroy viral DNA in the cytoplasm by cutting it. This process is comparable to a police officer searching for criminals with a wanted poster. When Cas9 encounters DNA within the cell that matches the gRNA - a criminal's face matches the poster - it cuts the viral DNA, effectively neutralizing the virus with a molecular death sentence.
The CRISPR-Cas9 system can be repurposed for genetic engineering by providing Cas9 with a specific gRNA. Instead of searching for viral DNA, scientists equip Cas9 with gRNA that matches a DNA sequence they wish to edit. Cas9 then locates this targeted section of DNA and creates a double-stranded break (a full cut of the DNA). However, this is only the first half of the gene-editing process.
Once CRISPR-Cas9 has cut the DNA the repair process begins. This is where genetic modifications are made. The two most common repair mechanisms used with CRISPR are Non-Homologous End Joining (NHEJ) and Homology Directed Repair (HDR). NHEJ is typically used for knockout modifications, where a gene is disabled, while HDR is used for knock-in modifications, where new genetic material is inserted. Both NHEJ and HDR are naturally occurring cellular processes that scientists harness to create genetic alterations.
NHEJ is a highly error-prone repair mechanism that often results in extra nucleotides, the building blocks of DNA, being inserted or deleted (indels). When indels occur within the coding region of a gene they inactivate the gene, creating a knockout. Knockouts can potentially be used to treat previously incurable viral disease, like HIV, by disabling infectious DNA within the patient. Additionally, researchers frequently use gene knockouts to study the function of genes, helping them understand the roles of specific genes in development and disease.
HDR, on the other hand, utilizes a template to repair the cut DNA. This template contains a gene of interest (GOI) flanked by regions of homology that match the sequences on either side of the cut. By providing a specific donor template, researchers can insert the gene of interest into the genome. This technique has many applications, including therapies for genetic disease and creating novel biological systems with synthetic biology.
The Business of CRISPR
CRISPR has revolutionized gene editing, making it cheaper and more efficient than ever before. Naturally, many companies have sprouted in this field. It is estimated that CRISPR has already enabled the creation of $32 billion in value from startups alone. The majority of these companies are nobly focused on developing treatments for previously incurable genetic diseases, offering new hope to patients with dire prognoses. However, CRISPR's applications extend beyond genetic disease. Featured below are some of the most unique and interesting CRISPR-based companies, how they are using CRISPR, the importance of their mission, and their current financial status.
Colossal Biosciences
Colossal Bio is using CRISPR to bring back extinct animals, specifically megafauna - the largest animals in an ecosystem - such as the woolly mammoth. They believe the effects will ripple down the food chain beginning to undo the damage of climate change. “Megafauna typically are apex predators and thus affect the entire food chain. They stomp out or eat invasive vegetation, pack certain harmful gases back into the turf, or in some instances kick up the earth and distribute nutrients. Their roles in the health of a given ecosystem can not be overstated,” explains Colossal on their website. The company plans to use CRISPR to knock-in mammoth genetic traits to Asian elephants, which already share approximately 99.6% of the mammoth's genome.
In addition to mammoths, Colossal is eyeing other extinct keystone species, such as the thylacine (Tasmanian tiger) and dodo bird. While their goals are ambitious, many question how this work will translate to revenue. Despite these concerns Colossal has raised significant funds.
Their latest round of financing was a massive $160 million Series B, resulting in a $1.5 billion valuation. The round was backed by some of venture capital's biggest names, including Tim Draper, Jim Breyer, and the CIA’s In-Q-Tel. Colossal CEO Ben Lamm recently stated to Semafor the appeal for investors is to invest in something that is "really cool for the world, that will spin off technologies that are good for the world, that will make history, and spin off technologies that are lucrative.” Colossal’s strategy of technology spinouts has been successful so far. Their first, Form Bio, raised a $30 million Series A and is now generating seven figures in annual revenue. Lamm hinted at a second spin-out in the works but did not provide further details.
eGenesis
You may have seen the recent groundbreaking news of a man who received a kidney transplant from a genetically engineered pig. The company behind this first-of-its-kind transplantation is eGenesis, a CRISPR-powered organ transplantation company. Key to the successful operation was the elimination of porcine endogenous retroviruses (PERVs) from the pig and organ prior to implantation. PERVs are infectious to humans and have caused the failure of past xenotransplantation efforts. eGenesis utilized the gene knockout power of CRISPR to remove PERVs, making the pig kidney safe for human transplantation.
Organ donor shortages are one of the most pressing crises facing the world today, with the number of candidates on the transplant waitlist growing much faster than the number of available organs. Currently, over 100,000 people are on the American national transplant waiting list, and 20 people die each day awaiting an organ transplant. Utilizing CRISPR, eGenesis is working to make these deaths a thing of the past.
The potential market for xenotransplanted organs has not gone unnoticed by investors. In 2021, eGenesis raised a $125 million series C funding round, achieving a $350 million valuation. They recently added another $25 million bridge round in early 2024. Although eGenesis’s HuCo (Human Compatible) organs are not yet publicly available, their liver is expected to enter clinical trials soon. They also have HuCo kidneys and hearts in their development pipeline.
Mammoth Biosciences
Co-founded by Jennifer Doudna herself, Mammoth Biosciences is one of the most noteworthy CRISPR startups. However, their CRISPR application may surprise you - Mammoth is leveraging CRISPR to diagnose disease. Their testing system, DETECTR, uses Cas12 and Cas13 rather than the more commonly known Cas9. These nucleases have unique properties that allow them to produce a visible signal when a target gene is identified. As co-founder Trevor Martin puts it to Synthego, “we started thinking about CRISPR being control F, rather than control X.” Essentially, using CRISPR as a search tool rather than a cutting tool.
Mammoth’s DETECTR system offers fast and accessible testing for infectious disease. The current gold standard in diagnostics, quantitative RT-PCR, typically takes 4-6 hours. However, as was seen during the COVID-19 pandemic, the actual time for patients to receive their results can be much longer due to sample transport and potential lab overload. This challenge is escalated in impoverished countries, where the high costs of PCR equipment limit access. CRISPR diagnostics provide an alternative that can be used at the point-of-care and deliver results in under 40 minutes. As the technology advances the cost of CRISPR-based tests is expected to decrease, enabling widespread distribution.
In addition to their diagnostic platform, Mammoth is actively researching new Cas nucleases and developing novel therapies that could provide permanent cures for diseases. Riding the hype wave of COVID, Mammoth had no trouble fundraising during the pandemic. They successfully raised $250 million across three rounds between 2020 and 2021, with notable investors like Apple CEO Tim Cook participating in their Series A. Currently, Mammoth is estimated to generate $35 million in annual revenue and has a $1 billion valuation. Despite the waning momentum in pandemic related diagnostics, Mammoth has continued to create value through other avenues. In April, they inked a five year $100 million deal with Regeneron to develop gene therapies.
Looking Forward
These are just a few of the revolutionary startups powered by CRISPR. There are also teams working to cure blindness and HIV, develop improved crops, and fight cancer. Remarkably, most of these companies are less than 10 years old! We are only witnessing the tip of a massive CRISPR iceberg.
As CRISPR technology advances the possibilities seem endless. But with great power comes great responsibility. How will we navigate the ethical challenges that come with editing the blueprint of life itself? Will reviving extinct animals restore our ecosystem, or trigger the chaos of Jurassic Park? One thing is certain: CRISPR is not just a tool; it is a force that will shape the future of our world.